 Science
Experiment "Phadung Krung Kasem Canal" Reconstruction
Science
Experiment "Phadung Krung Kasem Canal" Reconstruction Science
Experiment "Phadung Krung Kasem Canal" Reconstruction
Science
Experiment "Phadung Krung Kasem Canal" Reconstruction
First Experiment: Water transparent
Work-day: 16 November 2003
Perform Implement
1. Zakidisk brass plate 1 item
2. Waist rope 1 rope
Method Experiment
1. Bring brass plate of water first spot (Yaknopawong) observe in white precinct of brass plate.
2. Until we can’t to see it, already stretch out brass plate quantitively rope effect between plate to water surface spot and perform thus more 3 times.
3. Experiment more 2 place such as limited.
|
Experiment Place |
Transparent (Deeply to throw light upon) (Unit: cm.) |
|||
|
First time |
Second time |
Third time |
Average |
|
|
Yaknoppawong |
31.0 |
31.5 |
32.0 |
31.5 |
|
Saipanya School |
40.0 |
38.0 |
39.0 |
39.5 |
|
Behind Unit of Water Keep litter |
42.5 |
41.0 |
41.5 |
41.7 |
Experiment Conclude
To be arrange in transparent thus
· Yaknoppawong 31.5 cm
· Saipanya School 39.5 cm
· Behind Unit of Water Keep litter 41.9 cm
From information Back of keep garbage is most transparent so there precinct are good receive supervise but Yaknoppawong has lowest transparent.
Second Experiment:
Consider Acid-Base of water
Work-day: 23 November 2003
Perform Implement
1. Universal indicator 1 box
2. glass and cover 3 items
Method Experiment
1. Bring glass 3 items to dip up water from 3 places such as limit together with write name of place on glass.
2. Bring Universal indicator 1 sheet, already dip into glass number 1 and observe color of paper together with record.
3. Thus water in more glass also makes the same as.
Consequence Experiment
|
Place |
pH value |
Color |
|
Yaknoppawong |
7 - 8 |
Jude green |
|
Saipanya School |
6 - 7 |
Green mix Yellow |
|
Behind Unit of Water Keep litter |
7 - 8 |
Jude green |
Experiment Conclude
Standard on 5.0-9.0 so pH such as from each place have value straight to standard from environment committee.
Third Experiment: Consider of temperature
Work-day: 23 November 2003
Perform Implement
1. Thermometer
2. Pen
3. Ruler or straightedge
Method Experiment
1. Quantitative the weather temperature and record.
2. Make defect on Thermometer by quantitative form extremity to rise up 10 cm.
3. Dip Thermometer into water by defect make be sufficient with water surface wait until mercury constant already record.
Consequence Experiment
|
Place |
Time (24 hr.) |
Water (Unit: ºC) |
Weather (Unit: ºC) |
Deeply (Unit: cm.) |
|
Yaknoppawong |
14.50 |
29.0 |
34.5 |
10 |
|
Saipanya School |
15.00 |
28.5 |
30.0 |
10 |
|
Behind Unit of Water Keep litter |
15.20 |
29.0 |
32.5 |
10 |
Experiment Conclude
Forth Experiment: Found Oxygen value in Phadung Krung Kasam Canal
Work-day: 12 December, 2003
Precinct: Saipanya School
Purpose
1. Able to fine Oxygen value from chemical experiment.
2. To investigate canal thus to rule over.
Perform implement
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11. Calculator 1
Solution
1. MnSO4 2.2 mol/dm3 2 cm3
2. KI 1 mol/dm3 2 cm3
3. H2SO4 concentrated 2 cm3
4. Na2S2O3 0.005 mol/dm3 100 cm3
5. Powder 0.5 g 1 cm3
6. NaOH 12.5 mol/dm3 2 cm3
To prepare solution
A
chemical formula
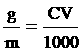
MnSO4 quantity
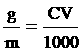
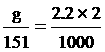
g = 0.7 gram fill water 2 cm3
KI quantity
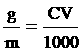
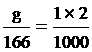
g = 0.3 gram fill water 2 cm3
Na2S2O3 quantity
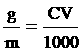
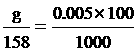
g = 0.08 gram fill water 100 cm3
NaOH quantity
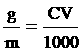
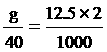
g = 1 gram fill water 2 cm3
Warning: Add acid to water must not add water to acid
Bring each solution into bigger 40 cm3 3 items and quantity bottle 2 items.
Method Lab
1. Keep (ex) water 250 cm3 in quantity bottle also close lid.
2. Fill MnSO4 2.2 mol/dm3 2 cm3 in (ex) water and close lid.
3. Fill KI 1 mol/dm3 in NaOH 12.5 mol/dm3 already absorb solution into measure cylinder 2 cm3 also fill into (ex) water close lid and wait to precipitate.
4. Bring H2SO4 intent 2 cm3 into (ex) water and reverse bottle.
5. Bring solution from No 4. quantity 50 cm3 into bottle size 100 cm3, drop Na2S2O3 0.005 mol/dm3 until solution become yellow color.
6. Fill powder water 1 cm3 for use indicator and tritrate also solution Na2S2O3 until solution no color.
7. Record all Na2S2O3 quantity.
8. Compute.

Consequence experiment thus
When fill MnSO4 in bottle of (ex) water has born yellow solution.
When bring KI + NaOH already fill solution into bottle of (ex) water also reverse bottle until born precipitate.
When fill H2SO4 find out that has higher temperature and lose brown precipitate.
Bring (ex) water from mentioned 50 cm3 fill in bottle size 100 cm3 already drop Na2S2O3 into bottle until yellow of water fade.
When fill powder water for use indicator find out that solutions of (ex) water become blue color and blue precipitate. Already fill tritrate also Na2S2O3 until solution no color.
Na2S2O3 quantity = 4.2 cm3 (Able made from Na2S2O3 as remain pour off 90 cm3 remain 10 cm3 fill in measure cylinder find out that solution remain Na2S2O3 5.8 cm3 so all remain 95.8 cm3. Bring 100 cm3 – 95.8 cm3 = 4.2 cm3)
When know all Na2S2O3 quantity…
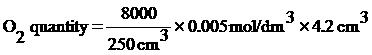
= 0.672 mg/dm3
Arising from water in canal shouldn’t have Oxygen lower 4 mg/dm3. But oxygen in water of canal in front Saipanya School is 0.672 mg/dm3. So water in canal in front Saipanya School has lower oxygen.
Reference: Chemistry Book No. 4 Ç.033, 2524